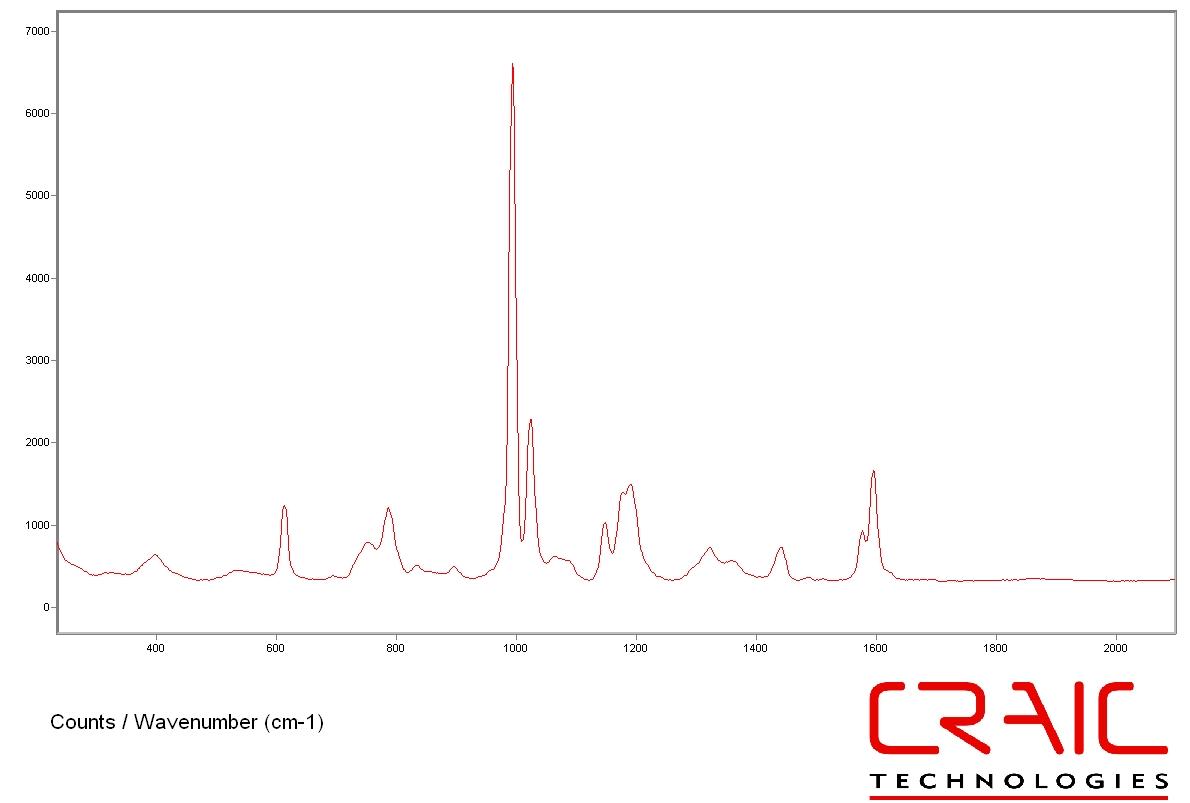
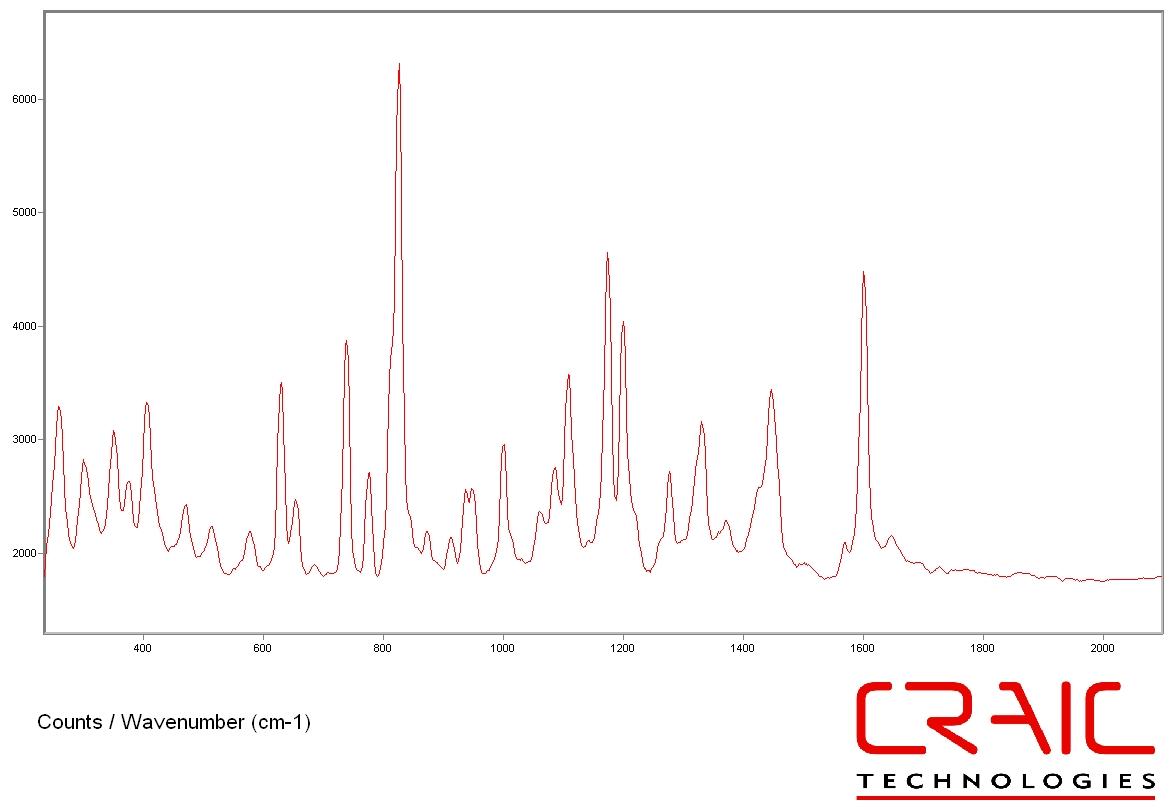
Science of micro Raman spectroscopy
Micro Raman spectrometer design
Applicationss of micro Raman spectrometers
Apollo M MicroRaman Spectrometer
Energy Level Diagram Depicting Raman Scattering
When photons interact with matter, such as when light is focused onto a sample in a microscope, it can either be reflected, absorbed or it can be scattered. We are interested in this last for the purposes of this tutorial.
The Raman Effect
Raman spectroscopy is the study of the interaction between light and matter in which the light that is inelastically scattered: a process called the Raman effect.
In a Raman spectroscopy experiment, photons of a single wavelength (in the visible range this would be light of a single color) are focused onto a sample. Most commonly a laser is used as it is a powerful monochromatic source. The photons interact with the molecules and are either reflected, absorbed or scattered. With Raman spectroscopy, we study the scattered photons.
Photons interacting with molecules most commonly scatter elastically. This is called Rayleigh scattering. Rayleigh scattered photons have the same wavelength as the incident light. However, approximately 1 out of a million photons are inelastically scattered...an effect first described by Sir Chandrasekhara Raman in 1922.
With Raman scattering, the incident photon interacts with matter and its wavelength is either shifted lower or higher (red or blue shifted, respectively). Red shifted photons are the most common, having been subject to a "Stokes shift". What has happened is that the photon has interacted with the electron cloud of the functional groups bonds, exciting an electron into a virtual state. The electron then relaxes into an excited vibrational or rotational state (see the diagram). This causes the photon to lose some of its energy and is detected as Stokes Raman scattering. This loss of energy is directly related to the functional group, the structure of the molecule to which it is attached, the types of atoms in that molecule and its environment.
Of course, not every molecule or functional group exhibits Raman scattering. Factors such as the polarization state of the molecule (which determines the Raman scattering intensity) must be considered. The greater the change in polarizability of the functional group, the greater the intensity of the Raman scattering effect. This means that some vibrational or rotational transitions, which exhibit low polarizability, and will not be Raman active. They will not appear in a Raman spectra.
Resonance Raman Spectroscopy
It should be noted that Raman scattering is a very weak effect as most photons are Rayleigh scattered. However, the intensity of the effect can be dramatically increased with resonance Raman spectroscopy. In resonance Raman spectroscopy, the wavelength of the exciting laser light coincides with the absorbance maximum of the molecule or functional group. Therefore, the photon can excite an electron to near an electronic excited state rather than a virtual excited state. This results in an increase in the Raman scattering intensity by a factor up to a million. This transition is therefore dominant in the spectra: the Raman spectra is of the molecule whose absorbance corresponds to the wavelength of the laser.


